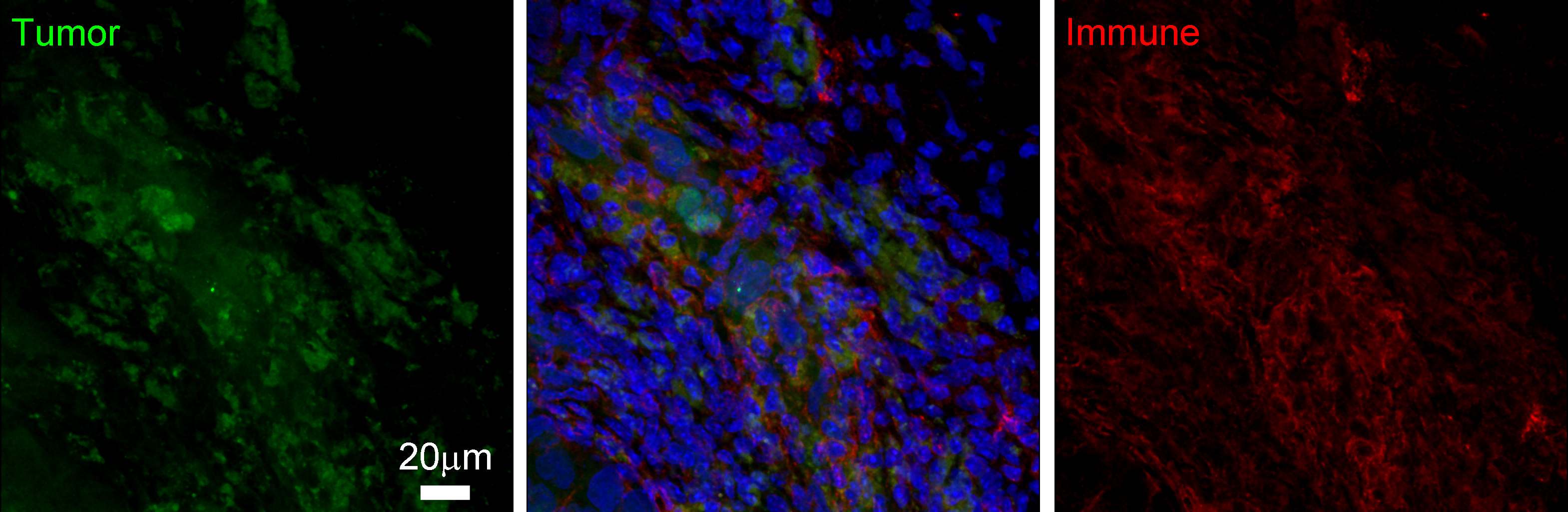
Immune cells can
infiltrate the cancer microenvironment, the consequences of which are not well
understood. The multi-faceted immune presence interacts with cancer cells in
inhibitory and/or stimulatory ways, resulting in complex cancer–immune
interactions. Below we describe our current mathematical and computational
approaches to understand the consequences and implications of these
intercellular interactions.
Tumor-Promoting
Inflammation
The presence of
cancer within a host initiates a systemic immune response towards the
transformed cells. Inflammatory immune cells such as neutrophils, platelets,
macrophages, and natural killer cells, are recruited to the tumor site where
they initiate the wound healing process. Tumors, sometimes viewed as wounds
that never heal, can be promoted by these inflammatory actions. Once the adaptive immune response is
activated by dendritic cells and macrophages, CD8+ T cells, or
cytotoxic T
lymphocytes, infiltrate the tumor and induce apoptosis in the target tumor
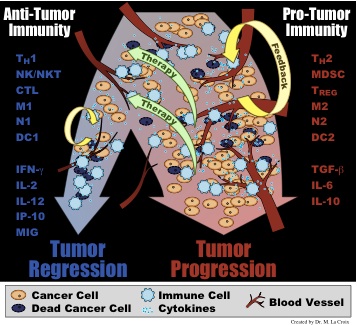
cells. Depending on the cytokines and other signals present in the tumor
microenvironment, recruited immune cells will either form a pro-tumor immunity
(typified by cytokines such as TGF-β, IL-6, and IL-10 and cells such as M2
macrophages, Th-2 T helper cells, and myeloid derived suppressor cells) or an
anti-tumor immunity (typified by cytokines such as IFN-γ, IL-2, and IL-12 and
cells such as M1 macrophages, Th-1 T helper cells, and cytotoxic lymphocytes).
To investigate the
role of tumor-promoting inflammation, an emerging hallmark of cancer, we have
developed a mathematical model for cancer-immune interactions that can capture
both the pro-angiogenic, tumor-progressing actions of a pro-tumor inflammatory
microenvironment, and the anti-angiogenic, tumor-inhibiting actions of an
anti-tumor inflammatory microenvironment. This model utilizes principles of
generalized logistic growth, which captures some of the inherent variability
underlying tumor growth in an immune competent host that is often neglected in
macroscopic measurements and in mathematical models. From model simulations,
the two types of inflammation (pro-tumor or anti-tumor) resolve into two
fundamentally different classes of outcomes, where inflammation-enhanced tumor
progression must either result in a decreased tumor burden, as in the
anti-tumor case, or in an increased tumor burden, as in the pro-tumor case.
These results suggest that, in some cases, fast tumor growth may be
advantageous, if it leads to a significantly smaller tumor burden. In such
cases, it is possible that treatments should be targeted towards enhancing the
stability of an anti-tumor inflammatory environment instead of towards
immediate tumor regression.
Tumor
Immunoevasion
Despite highly
evolved adaptive immune responses, tumors often manage to escape recognition by
the immune system. This process is known as immunoevasion, and is another
emerging hallmark of cancer.
When hematopoietic
stem cells leave the bone marrow they differentiate into either lymphoid
progenitors or myeloid progenitors. Lymphoid progenitors migrate to the thymus
where they differentiate into T, B, and NKT cells. Myeloid progenitors
differentiate into monocytes, migrate to tissues, and differentiate into
myeloid cells such as dendritic cells (DCs) and macrophages. When an immature
DC encounters an antigen, it internalizes the antigen to display fragments on
its membrane. The DC then matures as it migrates to a lymph node. Maturation
involves the loss of ability to engulf pathogens and an increased ability to
communicate with T cells. Within the lymph nodes (collection points where
antigen presenting cells interact with T cells attracted to the node via
chemotaxis), mature DCs activate naïve T cells to develop a specific immune
response. Activated cytotoxic T cells undergo rapid clonal expansion and
migrate throughout the body in search of relevant targets. T cells perform
their cytotoxic function by inducing apoptosis in the target cell through the
secretion of perforin and granzymes or through Fas/Fas-ligand binding.
Within the process
of activating the adaptive immune response described above, two significant
functions may be subverted by tumors: antigen presentation (maturation of DCs)
and T cell functionality.
Antigen
presentation suppression
If antigen
presentation is blocked then naïve T cells are not activated and a specific
immune response is not mounted. A number of cytokines, chemokines and growth
factors, such as HIF-1α, VEGF, nitric oxide, and reactive oxygen species (ROS),
produced within the tumor microenvironment may interfere with the process of DC
maturation. Without DC maturation, there may be an accumulation of
immunosuppressive factors, such as myeloid derived suppressor cells (MDSCs), in
the tumor microenvironment resulting in immunoevasion.
In order to
investigate this process from a modeling perspective, we formulated a system of
ordinary differential equations in a predator-prey type system, where the prey
(cancer cells) have a defense mechanism (immunoevasion) against recognition by
the predator (immune system). Our analysis suggests that this mechanism can
have significant effects on overall tumor-immune dynamics, ultimately allowing
for either tumor suppression or tumor escape in a manner that depends on the
strength of the immune suppression [Kareva et al, 2010]. Currently, we are
investigating the possible role of glycolysis and the resulting reduction of pH
in a hypoxic tumor microenvironment as another possible mechanism for immune
evasion.
Impaired
T cell functionality and immune resistance
The immune response
poses a second barrier to tumor growth after the angiogenic switch. Immune
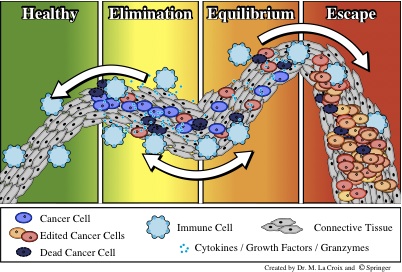
surveillance of tissues allows for early detection of transformed cells. If
these transformed cells are not recognized as “self”, they are eliminated by
the immune system. Through repeated exposure of the transformed cells to this
immune selection process, various phenotypes can arise within the cancer cell
population, creating a heterogeneous population of neoplastic cells. These
immunoedited cells may develop the ability to evade the immune response and
grow in an uncontrolled manner.
After prolonged
periods of immune-induced dormancy, T cells can lose effectiveness in their
cytotoxicity. This loss may be due to either T cell tolerization to the cancer
cells or to an increased cancer cell resistance to immune attack. Both of these
mechanisms are intertwined in the process of immunoediting that can lead to
tumor escape from immune control.
To investigate the
heterogeneous population-level dynamics involved in this immune selection
process, we are working on a mathematical model that can capture the essential
cancer-immune interactions that may lead to T cell tolerization and / or the
accumulation of immune-resistance by cancer cells. These two fundamentally
different mechanisms of immune evasion would require specifically targeted
therapies, which could be analyzed theoretically with this mathematical model.
Cancer
Stem Cells and Immune System-Modulated Tumor Progression
The role of the
immune system in tumor progression has been subject to discussion for many
decades. Numerous studies suggest that a low immune response might be
beneficial, if not necessary, for tumor growth, and only a strong immune
response can counter tumor growth and thus inhibit progression.
Without an immune
response, a heterogeneous tumor population comprised of cancer stem cells and
non-stem progenitors grows as conglomerates of self-metastases [Enderling etal., 2009]. This morphological phenomenon results from the interplay of cell
proliferation, cell migration and cell death. With increasing cell death
intra-tumoral spatial inhibitions are loosened, which in turn enable cancer
stem cell cycling and thus, counter-intuitively, tumor progression [Enderlinget al., 2009b]. By overlaying on this model the diffusion of immune reactants
into the tumor from a peripheral source to target cells, we simulate the
process of immune-system-induced cell kill on tumor progression. A low
cytotoxic immune reaction continuously kills cancer cells and, although at a
low rate, thereby causes the liberation of space-constrained cancer stem cells
to drive self-metastatic progression and continued tumor growth. With
increasing immune system strength, however, tumor growth peaks, and then
eventually falls below the intrinsic tumor sizes observed without an immune
response. Focusing only on the cytotoxic function of the immune system, we were
able to observe all immunoediting roles of the immune system: immune promotion
at weak immune responses, immunoinhibition at strong immune responses, and
immunoselection at all levels. Simulations of our model support a hypothesis
previously put forward by Prehn [Prehn, 1972] that comparable tumor sizes can be
observed for weak and strong immune reactions. With this increasing immune
response the number and proportion of cancer stem cells monotonically
increases, implicating an additional unexpected consequence, that of cancer
stem cell selection, to the immune response.
Cancer stem cells
and immune cytotoxicity alone are sufficient to explain the three-step
“immunoediting” concept — the modulation of tumor growth through inhibition,
selection, and promotion. We propose more generally that a stem-cell-expansive
influence may take the form of anything that encourages morphological
fingering. Beyond immune response, this could include cell death, or even
growth within restricted thin channels, as might be expected e.g. during
invasion of host tissue.see more......

Hey to everyone, it’s my first visit of the blog site; this blog includes awesome and actually best info for the visitors.
ReplyDeleteclick for more